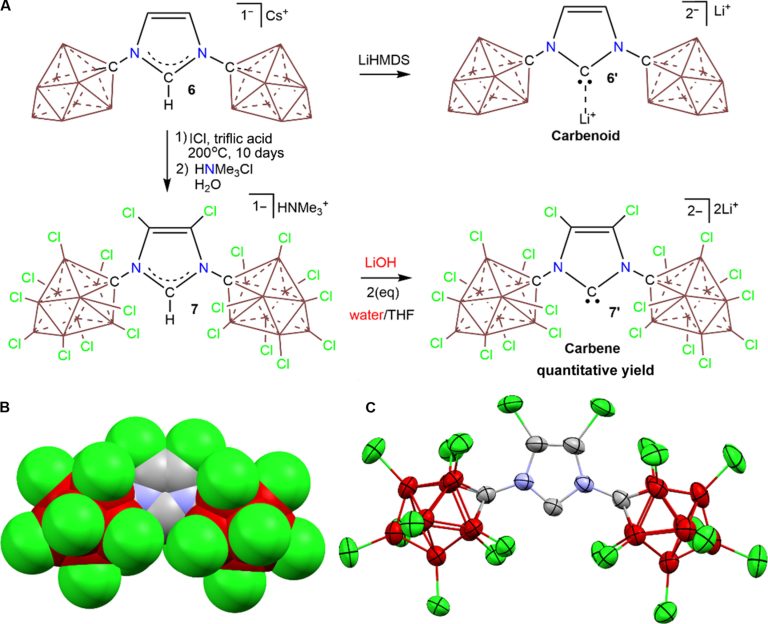
Summary and determination of 7 ′. (A) Summary of carbenoids 6 ′ and carben 7 ′. The reaction is clean and quantitative when LIHMDS are used as a base and pure THF as solvent. (B) Tidimensional space filling model. (C) Thermal ellipsoid layout attracted by the probability of 50%. Color code: C, gray; B, brown; N, blue; Cl, green. Note that two li (THF)4+ The counters are omitted for more clarity. Credit: Scientific advances (2025). DOI: 10.1126 / SCIADV.ADR9681
The chemists have confirmed a 67 -year theory on vitamin B1 by stabilizing a reactive molecule in the water – a long -term feat. The discovery does not only solve a biochemical mystery, but also opens the door to greener and more effective means of making pharmaceutical products.
The molecule in question is a carbenus, a type of carbon atom with only six valence electrons. Generally, carbon is stable with eight electrons around it. With only six electrons, it is chemically unstable and highly reactive. In water, it usually breaks down instantly. But for decades, scientists have suspected that vitamin B1, also known as thiamine, could form a carbenic type structure in our cells to make vital reactions in the body.
Now, for the first time, the researchers not only generated a stable carben in the water, they also isolated it, sealed it in a tube and watched it stay intact for months. This discovery is documented in an article published last week in Scientific advances.
“This is the first time that anyone who has been able to observe a stable carben in water,” said Vincent Lavallo, professor of chemistry at UC Riverside and corresponding author of the newspaper. “People thought it was a crazy idea. But it turns out that Breslow was right.”
The reference is to Ronald Breslow, a chemist from Columbia University who proposed in 1958 that vitamin B1 could convert to carben to conduct biochemical transformations in the body. Breslow’s idea was convincing, but carbens were so unstable, especially in water – that no one could prove that they really existed in a biological setting.
The Lavallo team succeeded by wrapping the carben In what he calls “a armor costume”, a molecule that they have synthesized in the laboratory which protects the reactive center from water and other molecules. The resulting structure is stable enough to be studied with Nuclear magnetic resonance spectroscopy And the X -ray crystallography – the conclusive proof of conclusive evidence that carbenes like it can exist in water.
“We were doing these reactive molecules to explore their chemistry, not pursuing a historical theory,” said the first author Varun Raviprol, who finished research as a graduate student at the UCR and is now a postdoctoral researcher at the UCLA. “But it turns out that our work ended up confirming exactly what Breslow proposed all these years ago.”
Beyond the confirmation of a biochemical hypothesis, discovery has practical implications. Carbens are often used as “ligands” or support structures, in metal -based catalysts – chemical horses used to produce pharmaceuticals, fuels and other materials. Most of these processes are based on toxic organic solvents. The method of carbenes stabilization researchers in water could help make these reactions cleaner, less expensive and safer.
“Water is the ideal solvent – it is abundant, non -toxic and environmentally friendly,” said Raviprol. “If we can make these powerful catalysts work in water, it is a big step towards greener chemistry.”
Know that such reactive intermediate molecules can be generated and survive in water also brings scientists closer to imitate the type of chemistry that occurs naturally in cells – which are mainly in water.
“There are other reactive intermediaries that we have never been able to isolate, just like this,” said Lavallo. “Using protective strategies like ours, we can finally see them and learn from them.”
For Lavallo, who spent two decades to design carbens, the moment is both professional and personal.
“Only 30 years ago, people thought that these molecules could not even be made,” he said. “Now we can bottle them in the water. What Breslow said all these years ago – he was right.”
For Raviprol, the discovery reminds of persevere in scientific research and discovery.
“Something that seems impossible today could be possible tomorrow, if we continue to invest in science,” he said.
More information:
Varun Tej Raviprol and Al, confirmation of the Breslow hypothesis: a stable carben in liquid water, Scientific advances (2025). DOI: 10.1126 / SCIADV.ADR9681
Supplied by
University of California – Riverside
Quote: Scientists finally confirm the hypothesis of vitamin B1 of 1958 (2025, April 21) recovered on April 22, 2025 from https://phys.org/News/2025-04-cscientist-vitamin-b1-hypothesis.html
This document is subject to copyright. In addition to any fair program for private or research purposes, no part can be reproduced without written authorization. The content is provided only for information purposes.