In this large prospective cohort study involving more than 50,000 participants, we identified 15 plasma metabolites associated with lifestyle factors, the majority of which were lipids. Through enrichment analysis, we hypothesize that lifestyle factors may influence the linoleic acid metabolism pathway and the glycerolipid metabolism pathway, thereby affecting overall health. Subsequently, we used accelerated time-to-failure models to explore the impact of various factors, including metabolites, on the latency period of chronic kidney disease (CKD). Our results provide new insights into the potential for early intervention and prevention of kidney disease, although further experimental and clinical studies are needed to validate these findings.
In our study, 15 plasma metabolites were associated with a comprehensive lifestyle, involving various fatty acids, lipoprotein subclasses and derived markers, such as saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, linoleic acid, triglycerides, HDL, LDL and VLDL, among others. Furthermore, previous studies have shown that plasma metabolites associated with different lifestyle combinations play a key role in elucidating the metabolic mechanisms by which lifestyle affects health. For example, a multicohort study identified metabolomic profiles from the Alternative Healthy Eating Index (AHEI), revealing robust and positive associations between AHEI scores and fatty acid unsaturation, as well as proportion and concentration of fatty acids. polyunsaturated fats, including omega-3 fatty acids (notably docosahexaenoic acid) and omega-6 fatty acids (notably docosahexaenoic acid) linoleic). Conversely, AHEI scores showed negative correlations with concentrations of saturated fatty acids and monounsaturated fatty acids.30. Another EPIC cohort study found that obesity can lead to alterations in metabolic profiles characterized by changes in urinary amino acids, sphingomyelins, glutamate, and various phosphatidylcholines.31. Additionally, other lifestyle factors, such as smoking32sleep33alcohol consumption34and physical activity35were associated with variations in amino acids, fatty acids, lipoproteins, and water balance metabolites, implicating the inherent metabolic effects of lifestyle behaviors on health status from a metabolomics perspective. Furthermore, based on our research results, we believe that lifestyle could mainly influence health by affecting lipid metabolism in the body. Anne-Julie Tessier et al. demonstrated in four large cohort studies that lifestyle metabolomic profiling reflected lipid metabolic pathways, thereby improving the predictive capabilities of overall mortality, cause-specific mortality, and longevity36. In a cohort study, researchers found associations between lifestyle factors and 81 plasma metabolites spanning diverse categories: lipids, lipoprotein subclasses, amino acids, fatty acids, ketone bodies, metabolism-related metabolites. fluid balance, glycolysis-related metabolites, and inflammation-related metabolites.37. Metabolites most significantly associated with lifestyle included concentrations of HDL particles, total choline, citrate, linoleic acid, omega-3 fatty acids, and phosphatidylcholine. Another cohort study from Spain found that an integrated lifestyle based on diet, physical activity, smoking, alcohol consumption and BMI led to changes in creatinine, acetone, citrate and certain lipid metabolites.38. Additionally, a separate UK Biobank study found associations between lifestyle and many lipid metabolites in plasma, including docosahexaenoic acid, omega-3 fatty acids relative to percentage of total fatty acids, monounsaturated fatty acids relative to the percentage of total fatty acids and linoleic acid relative to the percentage of total fatty acids. , align with the results of our present study19. Nevertheless, due to inconsistent definitions of lifestyle combinations and different coverage of measured metabolites in various studies, there may be heterogeneity in metabolic profiles. Further studies, such as standardized lifestyle definitions, plasma metabolite assessments, and long-term follow-up studies, are needed to provide a solid scientific basis for developing more effective health strategies. Additionally, because the lifestyle-related metabolites we identified are largely data-derived rather than direct metabolite concentrations, we selected key lifestyle-related metabolites for enrichment analysis, at instead of using derived indices. For example, we used linoleic acid rather than “percentage of linoleic acid to total fatty acids” for the enrichment analysis. Additionally, some metabolites are only labeled by broad categories (e.g., polyunsaturated fatty acids, monounsaturated fatty acids, saturated fatty acids) without a specific KEGG identifier, which limits precise identification of metabolic pathways. Therefore, future studies will need to perform more precise measurements of metabolites and further validation of metabolic pathways.
In this study, we used accelerated time-to-failure models to explore the factors influencing the onset of chronic kidney disease. Among traditional risk factors, smoking, hypertension, diabetes, and high BMI remain important predictors for the development of chronic kidney disease. These results highlight the importance of preventing CKD by prolonging the effective disease-free survival period, thereby emphasizing the need for appropriate management of chronic diseases and maintenance of healthy lifestyle habits. Additionally, using NMR-based metabolomics, we performed further analysis of plasma metabolites and observed an association between triglycerides present in large LDL particles and the onset of chronic kidney disease. This highlights the critical role of a low-fat diet and management of lipid levels in kidney health. Triglycerides found in large LDL particles are associated with accelerated progression of chronic kidney disease. The mechanisms by which triglycerides contained in low-density lipoproteins (LDL) contribute to kidney injury have become an important focus of research in the field of chronic kidney disease (CKD). Studies have shown that triglyceride-rich lipoproteins (TRL) are elevated in patients with CKD and may accelerate kidney damage through several pathways. First, elevated triglyceride levels are often accompanied by disturbances in lipid metabolism, leading to lipid deposition in renal tissues, particularly in the renal tubules and interstitial areas. These deposits can trigger localized oxidative stress, increasing the generation of reactive oxygen species (ROS), which in turn cause cellular damage and apoptosis.39. Additionally, increased triglycerides may alter the composition and function of lipoproteins, making them more likely to induce immune and inflammatory responses, thereby exacerbating local inflammation in the kidneys. The release of inflammatory cytokines such as TNF-α and IL-6 activates immune cells, further damaging renal tubular epithelial cells and promoting the progression of renal dysfunction.(45) Additionally, increased triglycerides can impact fatty acid metabolism, leading to elevated levels of free fatty acids in the kidneys. These free fatty acids can promote the fibrotic process in kidney tissues through various mechanisms, ultimately leading to further deterioration of kidney function. Specifically, triglycerides, by modifying lipid and fatty acid metabolism, activate signaling pathways linked to fibrosis, oxidative stress and inflammation, which contribute to structural and functional damage to the kidneys.40. Therefore, in managing kidney health, adopting a comprehensive lifestyle intervention is crucial. In addition to recommending at least 150 minutes of moderate-intensity aerobic exercise per week, maintaining a healthy BMI, ensuring 7-9 hours of quality sleep, limiting alcohol consumption and promoting cessation of tobacco, diet and blood lipid management play a key role. in the protection of renal function. A low-fat diet should prioritize sources of unsaturated fatty acids, such as fatty fish, fish oil, and vegetable oils. These foods not only provide healthy fats, but are also rich in omega-3 fatty acids, which help reduce inflammation, improve cardiovascular health, and support kidney function. On the other hand, the consumption of animal fats should be minimized, especially that of red meat, full-fat dairy products and fried foods, as these foods tend to increase blood lipid levels, promote oxidative stress and trigger inflammatory responses, which can accelerate kidney damage. . Additionally, adequate dietary fiber intake, a low-sodium diet, and sufficient hydration are also essential for maintaining optimal kidney function.
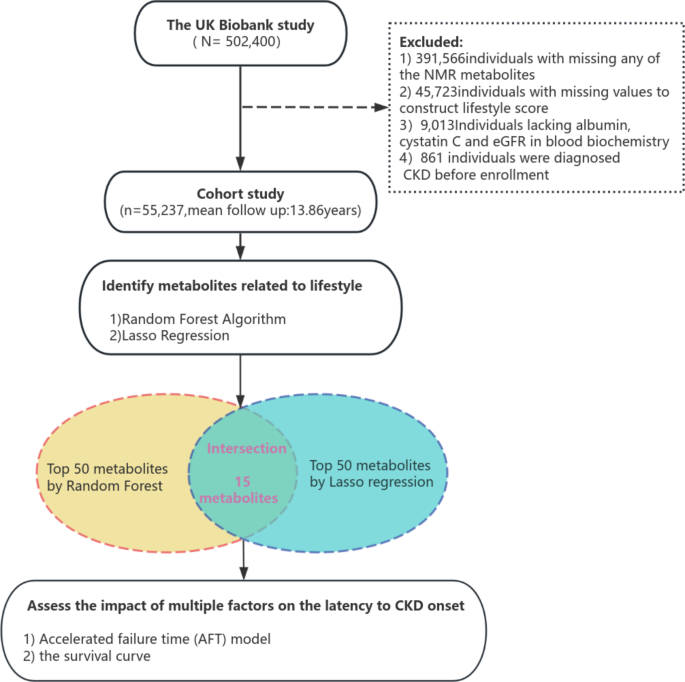
This study had several strengths, including a large sample size and a prospective cohort design. Additionally, we collected comprehensive participant lifestyle data from the UK Biobank to create lifestyle scores. Leveraging Lasso regression and Random Forest algorithm, we identified relevant metabolites and assessed their associations with CKD. Additionally, we used accelerated time to failure (AFT) models to study factors associated with acceleration or deceleration of the latency period of chronic kidney disease (CKD). However, several limitations should be noted: first, the lifestyle data were initially collected through surveys, physical measurements and self-reports, lacking information on potential changes in lifestyle. life during follow-up, which can introduce estimation errors for incident cases of CKD. Second, the generalizability of our results may be limited due to study-specific constraints. The survey was limited to the United Kingdom, with predominantly white participants aged 40 to 69. Dietary and lifestyle factors in this population may differ from those in other regions and ethnic groups. Additionally, we lacked data on the incidence of CKD in other age groups or healthy populations, which could offer additional insights.