There is an unexpected movement in the world of cell biology – in particular, with the energy factories called mitochondria.
Since they were discovered in the middle of the 19th century, mitochondria have been known as organelles that reside within cells. But this manual image now seems bad. An explosion of research questions the long -standing image of mitochondria as exclusively cellular organelles. “It can be a multicellular ordeal,” explains Jonathan Brestoff, an immunologist who studies metabolism at the University of Washington in St. Louis, Missouri. In other words, supposedly static energy factories now seem to be expert travelers, Go from one cell to another on request.
“Poison” cancer cells The immune system with contaminated mitochondria
This “mitochondrial transfer” was observed in a wide variety of cells and in organisms as diverse as yeast, molluscs and rodents. “It’s really exciting to see,” explains Jeffrey Spees, biologist of STEM cells at the University of Vermont in Burlington.
We do not yet know why the mitochondria are so mobile. Some studies have suggested that cells give their mitochondria to their neighbors if necessary. In cellular emergencies, newly arrived mitochondria could launch tissue repair, light the immune system or save the cells in death from death. Other research suggests that mitochondrial transfer can be a deadly weapon that Cancer cells are deployed to obtain an advantage.
But what it means for human health is always a mystery. The researchers have not yet captured the process inside the human body and therefore do not know with certainty if it happens in people, explains Daniel Davis, an immunologist at the Imperial College in London. “We don’t have technology yet to attend what’s going on,” he says.
This fact did not prevent researchers from exploring how to take advantage of the mitochondrial transfer to treat a variety of diseases, including cancer and cerebral vascular accidents.
A borrowed bacteria
Over the past three decades, research has revealed that mitochondria is much more than cellular powers that transform food nutrients into energy. These are key players in several body processes: they guide the chatter that maintains the functioning of cells and contributes to the immune response against harmful invaders. They are also unexpected. Last year, researchers discovered that mitochondria divide into two distinct forms To help cells survive nutritional famine1. Another study, published in March, modeled Density and type of mitochondria throughout the human brain2.
All the mitochondria – in whatever the organism, in whatever the part of the body – would have come from the same old bacteria. About 1.5 billion years ago, this drifting bacteria was swallowed by the microbe which finally gave birth to the eukaryotes – the large group of organisms, whose cells have a closed nucleus.
Scientists make precise gene changes to mitochondrial DNA for the first time
After several scalable twists and turns, this The borrowed bacteria has become the organic which leads to metabolism. The microbial origins of the mitochondria probably help to explain why they are more dynamic than they seem first, explains Kazuhide Hayakawa, neuroscientist at the General Hospital of Massachusetts in Boston, which studies how the mitochondrial transfer could help treat brain vascular accidents. “It is possible that the mitochondria keep the old ability to spread from cell to the other, such as bacteria,” he said.
In 2006, Spees and his colleagues captured the first overview of mitochondria from one cell to another3. The team had tried to understand a perplexed behavior of stem cells in laboratory dishes. These cells seemed to share a kind of physical information that told them how to differentiate themselves, and the mitochondria were considered to be involved.
To study the role of mitochondria, researchers cultivated cancer cells from the human lung, which did not have organelles, with stem cells from bone marrow. With the mitochondria of stem cells marked with fluorescent proteins, the team turned an accelerated video of what happened next.
Black and white granular images showed that stem cells were pulling their mitochondria, which were then retained by defective pulmonary cells. After the donation, pulmonary cells quickly recovered their ability to divide and transform glucose into energy. “Watching it was like a miracle,” said Spees.
Since then, researchers have observed zipping mitochondria between several types of cells – lung, heart, brain, fat, bone and more. Sometimes the mitochondria move on the ephemeral highways known as tunnel nanotubes which are formed between the cells and transport other cellular cargoes. In other cases, mitochondria make their trip to bubble -shaped vesicles or float freely in the blood4 (See “Three ways to travel”).
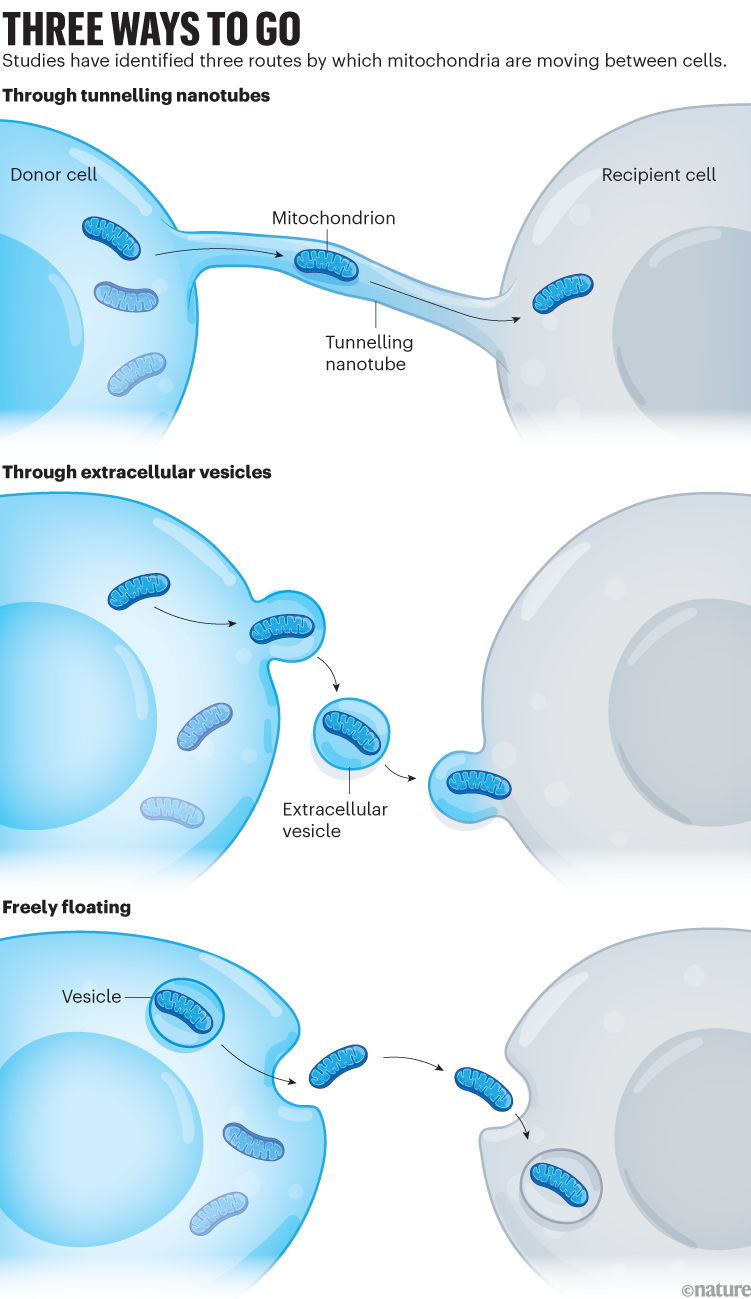
The way the mitochondria move is largely resolved, but what is less clear is why. Researchers learn that the process is often a form of cellular damage control, explains Clair Crewe, a cellular biologist at Washington University.
Some studies suggest, for example, that mitochondrial transfer could help cells resist neurological storms. In 2016, Hayakawa and his colleagues discovered that in mice who had a stroke, Cell support called astrocytes Deliver their mitochondria to flicker neurons. With this mitochondrial boost, neurons increased branches and restarted their metabolic processes, which has improved their chances of survival. When the researchers inhibited mitochondrial transfer, fewer neurons have survived, suggesting that the ordered organelles were essential to the recovery of cells5. But what part of the structure or function of the mitochondria protected the cells remains unknown, explains Hayakawa.
Pulmonary cells could also benefit from a mitochondrial boost during a crisis, explains Jahar Bhattacharya at Columbia University in New York, specializing in severe inflammatory disease known as acute pulmonary lesion. He and his colleagues discovered that in mice with this inflammation, the stromal cells – which make up the connective tissues that support organs – transfer their mitochondria to pulmonary cells6. Cells with lent or so or more high concentrations in cellular fuel ATP, which ended up being distributed to neighboring cells that have not received new mitochondria.
These sick lungs have shown more signs of recovery than sick lungs that have not received outside the mitochondria. Bhattacharya was amazed when he and his team witnessed the mitochondrial transfer to action. “I don’t think we slept for the next nights, it was so exciting,” he said.
Other research advice that transferred the mitochondria could overeat the healing of wounds. In 2021, Anne-Marie Rodriguez, cell biologist at the University of Sorbonne in Paris, and her colleagues discovered that the isolated plates of human blood closed their mitochondria to stem cells when the researchers put the two types of cells in a dish. After taking the mitochondria, stem cells released molecules that play a role in the formation of new blood vessels. When the cells were placed on skin wounds in mice, these injuries healed faster than wounds in rodents who had received stem cells or plates alone7.
Researchers suspect that cells with dysfunctional mitochondria may even have ways to ask for healthy mitochondria from their neighbors, although the exact mechanisms underlying this process remain troubled. “We are only starting to understand the signaling involved,” says Crewe.
Daily operations
Beyond its role in the recovery, researchers want to know if mitochondrial transfer is an essential element of daily biology. The first evidence suggests that this could help maintain healthy tissues. Last year, Minghao Zheng, regenerative biologist at the University of Western Australia in Perth, and his colleagues discovered that certain types of astrocytes gave their mitochondria to cells that line the blood vessels in the brain of the mouse8. When the researchers disrupted this process, the blood-brain barrier became fleeing, which suggests that the mitochondrial transfer helps maintain this protective membrane shield. Zheng and his team had already pointed out that the mitochondrial transfer in the mouse bones can accelerate the formation of new blood vessels9.
In healthy mice, Brestoff and his colleagues reported that white fat cells transfer their mitochondria to macrophages – white blood cells that aspire cell debris. The number of ordered ordered organizations has been reduced in obese mice. Obese mice have also burned less energy than their healthy counterparts10. These organelles could help macrophages work when their metabolism is disturbed, explains Brestoff.
In the labyrinthine world of the immune system, data mitochondria could have an anti -inflammatory effect, especially when absorbed by T lymphocytes – White blood cells stifling infections and disease. In studies in cell cultures, Patricia Alejandra Luz-Crawford, immunologist at the Andes University in Santiago, Chile, and her colleagues have found that certain types of T cells that receive mitochondria of stem cells produce fewer inflammatory molecules. Stem cells cultivated with people with rheumatoid arthritis transmit fewer mitochondria to T cells than stem cells of healthy individuals, which, according to her, could contribute to chronic inflammation associated with the disease11.
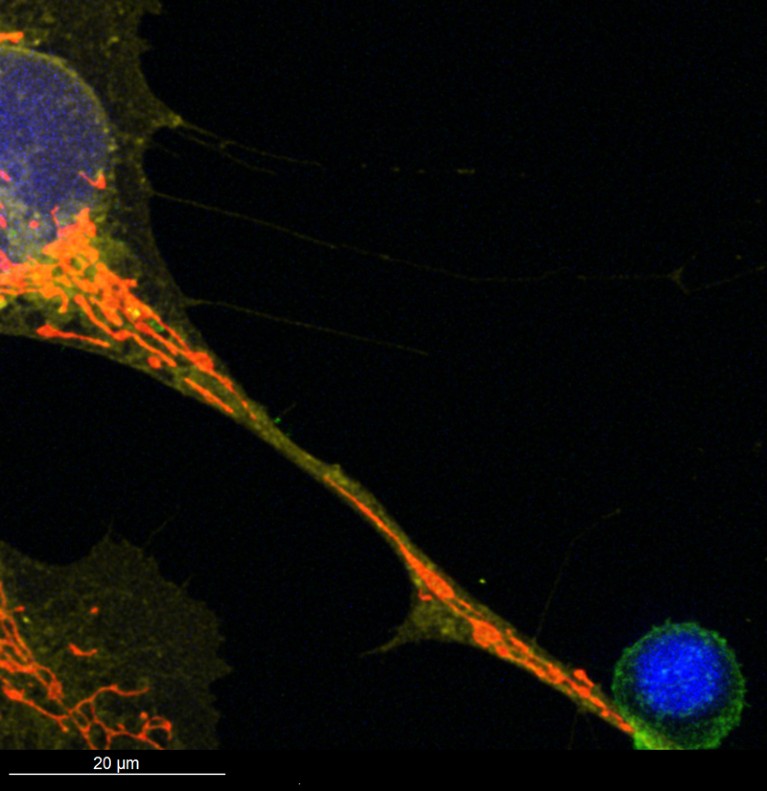
The mitochondria, visible in red, travel through a tunneling nanotube of a type of bone marrow cell (top left) to a T cell that fights infections and cancer (bottom right).Credit: Jeremy Baldwin
But there are a lot of unanswered questions about the mitochondrial transfer, including what organizations could do after entering the cells and how long they last, explains Luz-Crawford. “There is still a lot of mystery.”
The lack of details on the reasons why cells transfer their mitochondria makes it difficult to know what specific role these cellular exchanges could have in conditions such as cardiovascular disease and obesity, explains Rodriguez. In vivo Studies have followed the mitochondria in only a handful of tissue types, which makes it difficult to build a wider image of the wider effects than these transfers are on health.