
Barry Datlof, Center, Head of Development and Marketing of Business at the Office of Medical Technology Transfer of Medical Research of Research and Development, poses with General James E. Rainey, on the right, General Commander of the Future Army Command and Stephen Luckowski, Director of the Technology Transition Transition and Office of the Commercial Partnership at the Deputy Secretary of Defense Secretary for Research and Technology during the reception of the Prize for the Defense Ministry 2024 George F. Linsteadt Technology Transfer ACHIENE AWARD January 30.
See the original
Fort Detrick, MD. – For 25 years, the medical transfer office office worked with inventors, companies and investors to market and deliver dozens of innovative devices and drugs for Warfighter and the public. The heart of MTT’s success is a award -winning process for systematic maturation and deactivation of first generation biomedical technologies called assistance technology transfer, or AT2. For its contributions to the development and implementation of the AT2, Barry Datlof, head of development and marketing of MTT affairs, recently received the George F. Linsteadt Defense Technology Transfer Prize Bureau of the Defense Under-Secretary for Research and Engineering.
Datlof accepted the prize on behalf of the MTT team during a reward ceremony on January 30. The annual price recognizes DOD employees who have found innovative means to accelerate the transfer of technologies developed within the DOD for commercial use in military and civil applications. Earlier this year, the Federal Laboratory Consortium for Technology recognized the MTT team’s AT2 program with a 2024 technology transfer innovation price.
During the award ceremony, Stephen Luckowski, Director of the Transitional Transition Office and Technology Commercial Partnership at the Deputy Secretary for Defense Secretary for Research and Technology 20 Million In income and 40 million Dollars in cooperative research and development agreements.

Barry Datlof, Head of Development and Marketing of Affairs to the transfer of the Office of Medical Research and Medical Development, explains how MTT uses its award -winning assistance technology transfer process to help inventors systematically mature and Disable first -generation biomedical technologies. Datlof recently received the Prize from the Defense Ministry 2024 George F. Linsteadt Technology Transfer Achievement Award of the Defense Subsecretaire office for research and engineering in recognition of its role in the development and implementation of the process at2.
See the original
“I know excellence when I see it,” said Luckowski. “You support the Warfighter mission in many ways. What could be better than sending a soldier home to their families? You provide technology for this, and you provide motivation to that. »»
More than 30 biomedical technologies have been successfully authorized using AT2, generating more than $ 26 million in royalties that MTT used to reward inventors and invest in the AT2 program to help move others New inventions through the development cycle, creating what MTT calls a successful loop comment. AT2 links inventors to expert assistance at each stage of development, helping to access funding sources, to meet experts who can answer questions about regulatory and license requirements, to obtain resources to develop and test Prototypes and help with military and civilian sales and licenses, among other supports. This ensures that new technologies will be mature and ready for manufacturing, increasing the probability that they are under license and accessible to DOD to purchase.
“The transfer of assistance technology is a culmination of recognition that it takes more than patents and licenses to align a real product,” explains Datlof, who invented the term ten years ago. “We have many assets in MRDC which allow us to create well -trained product portfolios which are attractive for potential licensees. For example, through our office of regulated activities, we can work with the American Food and Drug Administration to conduct clinical trials. We have access to large research and development facilities such as laboratories and additive manufacturing for the development of prototypes. We can put devices in the hands of combatants and doctors to test them in real conditions. And we have access to several sources of funding to pay for these activities. All this represents millions of dollars and countless hours of work that a licensee does not have to invest alone. “”
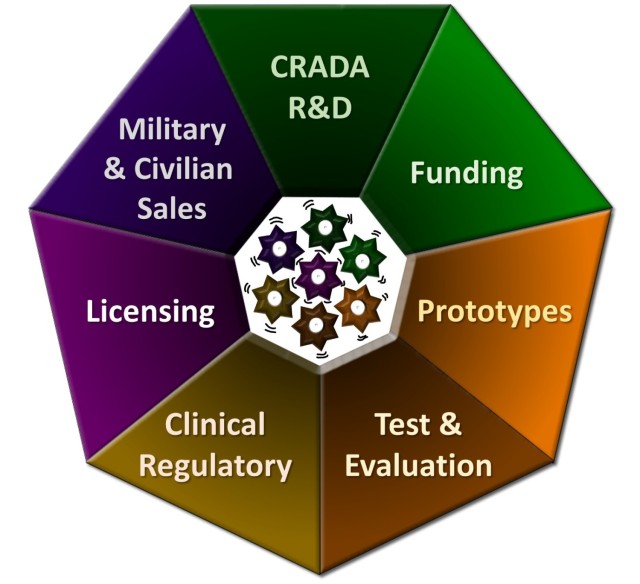
The assistance technology transfer process developed by the medical transfer of medical research technology and medical development connects inventors to expert assistance at each stage of the development of their inventions, especially during the first critical stages Prototyping and field tests. This illustration shows the seven areas of intervention discussed by the AT2 process, which helps to guarantee that new technologies will be mature and ready for manufacturing, increasing the probability that they are under license.
See the original
Another unique asset that MTT brings to the transfer of technology is its team of experts, supplementing traditional technology transfer entrepreneurship with what Datlof calls “intra-prienurship”.
“We collect a team of people with large history who are ready to ask:” What if? ” “Said Datlof. “Everyone has two, if not three areas of expertise. Taking risk is part of the equation we are looking for, as well as creativity. There are few schools focused on technology. Almost everyone in MTT received a cross training from the “Street University”.
The context of Datlof illustrates this diversity. A self-written “budding development biologist” he launched the Dana-Farber Cancer Institute technology transfer office in the 1980s and then did the same for the American Red Cross. He joined the Research and Material Equipment Transfer Office, when he wanted to work for its founder, Dr. Paul C. Mele. In the mid -90s, Mele, a former researcher of the company Philip Morris, testified before the Health Committee of the Chamber and the environment on the efforts of his former employer to remove research on the addictive properties of nicotine.
“This is the kind of legal person with whom I like to work,” explains Datlof. “I think it’s really important for most people who also work in technology transfer. The goal is important. “”
Due to the MTT collaborative approach, Datlof considers the Linsteadt Prize as recognizing the achievements of the whole team.
“Our team is really the prize winner because no person in our group knows each step, each nuance of the process,” explains Datlof. “It’s a real team effort. None of us steal solo, including me. »»
While the MRDC medical transfer of medical transfer is launched in its next 25 years, Datlof has many ideas on what the future can hold. It proposes the establishment of an “angel fund” at the DOD scale to accelerate the adoption of AT2 in smaller and more recent technology transfer offices. The team also studies the means to take advantage of digital market technologies to accelerate technology transfer via an online showcase similar to the new Space Force commercial acquisition portal. Datlof would also like the DHA to increase its international patent portfolio to stimulate risk capital investment and increase license income flows in several countries.
“Fortunately, it is something that I am addicted, just like my colleagues,” explains Datlof. “None of us want to leave because the level of gratuity when you saw that you have helped save someone’s life is immense. What should you not like work to do that?


